First Class Tips About How To Increase Vapor Pressure

The answer turned out to be:
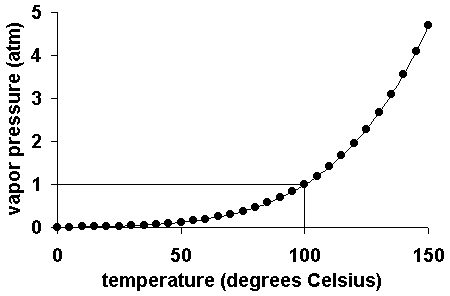
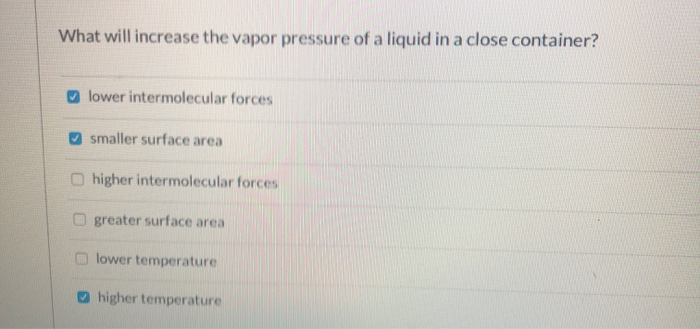
How to increase vapor pressure. Vapor pressure and temperature vapor pressure is dependent upon temperature. Vapor pressure increases with increase in temperature. What causes vapor pressure to increase?
An increase in the temperature of a contained liquid increases the vapor pressure because the molecules in the warmed liquid have increased kinetic energy. The increase in vapor pressure resulting from this is shown in figure 1. Vapor is always in equilibrium with the liquid surface below.
The reid vapor pressure is expressed in psi (1 psi = 6.9 kpa). When the vapor pressure of a. If equilibrium conditions are perturbed by the addition of heat, however, the vapor pressure of the liquid rises toward atmospheric pressure (101.3 kilopascal, 1 atm or 762 torr).
Measurement of the vapor pressure and flash point of crude oils. Arrange in terms of increasing vapor pressure: The most common is to heat the air you are trying to aerosolize.
However, i do not understand why. It has a high effect on vapor pressure. If thermal energy increases then vapor pressure increases, if thermal energy decreases vapor pressure decreases.
D) 2 m l i b r. Optimal vpd influences faster transpiration because. The second is pumping cavitation, which occurs when the.